Phosphorus trifluoride is a chemical compound with the chemical formula of PF3. PF3 is known as a ligand in metal complexes, and it has a high level of toxicity in nature.
To answer your question if Phosphorus Trifluoride is a polar or nonpolar molecule, our team did thorough research to provide you with all the information about the polarity of this compound.
Contents
Phosphorus Trifluoride: Is It Polar or Nonpolar?
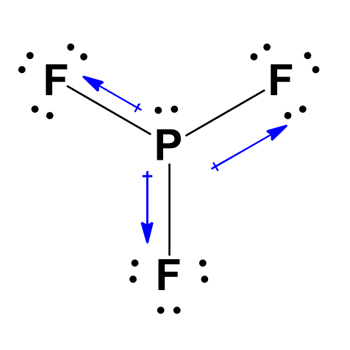
PF3 is a polar molecule. Due to the one lone pair present in the central atom Phosphorus, it generates repulsion. Nonpolar molecules have zero dipole moment, while polar molecules have greater than zero dipole moments. PF3 creates a dipole moment of 1.03 D which is directly proportional to the entire molecule.
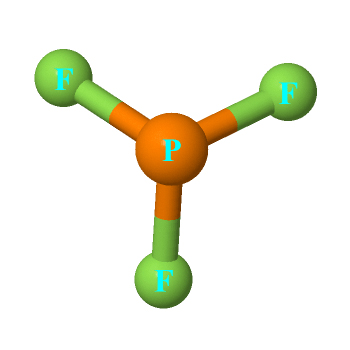
The central atom of the PF3 molecule is Phosphorus, which is surrounded by three fluorine atoms. To complete their octet, all three atoms of fluorine share one electron of Phosphorus, keeping one lone pair on the phosphorus atom.
The electronegativity difference of PF3 also determines that it is a polar molecule. The more electronegative atom attracts the charge, making it polar.
Name of Molecule | Phosphorus Trifluoride |
Molecular Mass | 87.96 g/mol |
Dipole Moment | 1.03 D |
Bond Angle | 96.3° |
Hybridization | sp3 |
Molecular Geometry/Shape | Trigonal Pyramidal |
Understanding Its Properties
The PF3 molecule is an odorless gas that reacts a tad slower along with water. Compared to other chemical compounds, it has a slow hydrolysis rate. PF3 has a molecular mass of 89.96 g/mol, a boiling point of 101.8 °C, and has a melting point of 151.5 °C. The outermost shell of fluorine has seven valence electrons, while Phosphorus has five valence electrons. The geometrical shape of PF3 is trigonal pyramidal.
According to VSEPR theory, polar molecules are due to the lone pairings that generate repulsion. This polar chemical is a gas that is very poisonous [1]. Because it is intensely poisonous, inhaling it is lethal, and exposure can result in extreme skin burns and damages to the eye.
Uses of PF3
In most metal complexes and metal carbonyls, this chemical is known as a ligand. It’s a chemical that binds the core atom P to build a stable complex.
When metals and carbon monoxide combines to form a new substance, carbonyls are useful in inorganic processes such as hydroformylation.
What Determines PF3 Polarity

PF3 Electronegativity
Compared to a less electronegative atom, the more electronegative atoms draw the bonded electrons pair with higher force. In terms of PF3 electronegativity, fluorine possesses a stronger electronegative atom than Phosphorus.
If two atoms have an identical amount of charge, their bond becomes nonpolar. Hence the two poles will receive a partial negative charge and a likewise partial positive charge.
Fluorine atoms form an electronegativity of 3.98, while Phosphorus is 2.19. The bonded atoms are polar because of their electronegativity. As a result, all P-F bonds have a non-zero dipole moment in the same direction.
Related Posts:
- CH2Cl – Is It Polar or Not?
- NH3 – Is It Polar or Not?
- PH3 – Is It Polar or Not?
- XeF4 – Is It Polar or Not?
- BrF3 Molecular Geometry
PF3 Geometric Shape
According to VSEPR theory, the PF3 molecule created a geometrical shape of a trigonal pyramid. An asymmetrical angle of roughly 96.3° will result from the two polar atom bond pairs. A nonpolar molecule has a symmetric structure, whereas PF3 has an asymmetric shape, which makes it a polar molecule.
PF3 Dipole Moment
Because PF3 does not contain hydrogen molecules, it is incapable of establishing oxygen and hydrogen bonds. Because PF3 contains lone pairs, there will be a non-zero dipole moment to the entire molecule, causing the compound to be polar.
The dipole moment is directly proportional to the molecule’s polarity; nonpolar molecules have zero dipole moments. Furthermore, dispersion forces will exist between molecules, resulting in a permanent dipole of polar particles.
FAQs
Pf3 is not an electrolyte. Covalent compounds don’t have ions to transport the electrical charge, and electricity cannot conduct well.
A covalent bond formed by two atoms is said to be polar if the electronegativity of the atoms differs. The more electronegative atom attracts the bonded charge towards itself, gaining a negative charge.
The chemical compound PF3 is under a covalent bond. After establishing three single bonds, Phosphorus will have a lone pair, which will intensify the attraction of both the two bonded pairs.
The greatest example of an ionic is sodium chloride. Because there will be no electron transfer, it possesses covalent bonds like Phosphorus Trichloride and Silicon Tetrafluoride
PF3 is a covalent compound because it is composed of P and F atoms. Each F atom shares one electron with Phosphorus, filling the octet of each atom. [2] Among all bond forces, covalent and ionic bonds have the greatest strength. Covalent bonding exists in polar molecules.
Final Thoughts on the Polarity of PF3
Phosphorus Trifluoride is a polar molecule. It is a chemical compound with three fluorine atoms and one on the phosphorus atom. The three Fluorine atoms are connected with Phosphorus, leaving one lone pair of electrons to complete the polar octet rule.
Because of the difference in their electronegativity, PF3 forms a geometrical shape of a trigonal pyramidal shape. PF3 is a polar molecule since the polar bond polarization and the moment are both non-zero.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://pubchem.ncbi.nlm.nih.gov/compound/Phosphorus-trifluoride
- https://study.com/academy/answer/is-the-compound-pf3-ionic-or-covalent-explain.html
- 8 Best Books On Evolution (2023 Updated) - May 16, 2022
- How Could Natural Selection Lead To Evolution? (2023) - May 16, 2022
- How Do Fossils Provide Evidence For Evolution? (2023) - May 16, 2022

