Dichloromethane, often known as methylene chloride, is an organic chemical compound. DCM’s chemical formula is CH2Cl2. It is an uncolored and volatile liquid with a slightly sweet odor.
But aside from this little background about Ch2Cl2, have you ever known if Ch2cl2 is a polar or nonpolar molecule?
And to answer that, our team could help you with the information we gathered below.
Contents
Dichloromethane (CH2CL2): Polar or Nonpolar?
Dichloromethane CH2L2 is a polar compound because of its tetrahedral structure. The molecules are arranged wherein the Carbon atom is in the middle, one Hydrogen atom is at the top, and the other is on the left side of the central atom. Because they both have four bonding pairs and no nonbonding pairs of electrons around the central Carbon, CH2Cl2 will have a molecular geometry of tetrahedral form. Hence, the shape is trigonal bipyramidal.
On the other hand, The polarity of any compound is determined by the number of lone pairs of electrons and the complex’s uniformity.
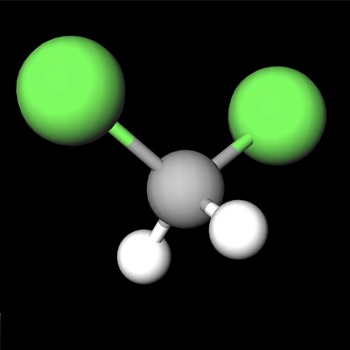
It is also identified by the electronegativity of the molecules involved in the compound’s formation. The compound has a net dipole moment because the hydrogen atom is less electronegative than the chlorine atom. Dichloromethane is also polar due to the asymmetric arrangement of the bonded pairs.
Name of Molecule | Dichloromethane or Methylene Chloride |
Molecular Mass | 84.93 g/mol. |
Dipole Moment | 1.67 D |
Bond Angle | 109.5° |
Hybridization | sp3 |
Molecular Geometry/Shape | Trigonal bipyramidal |
Its Molecular Structure
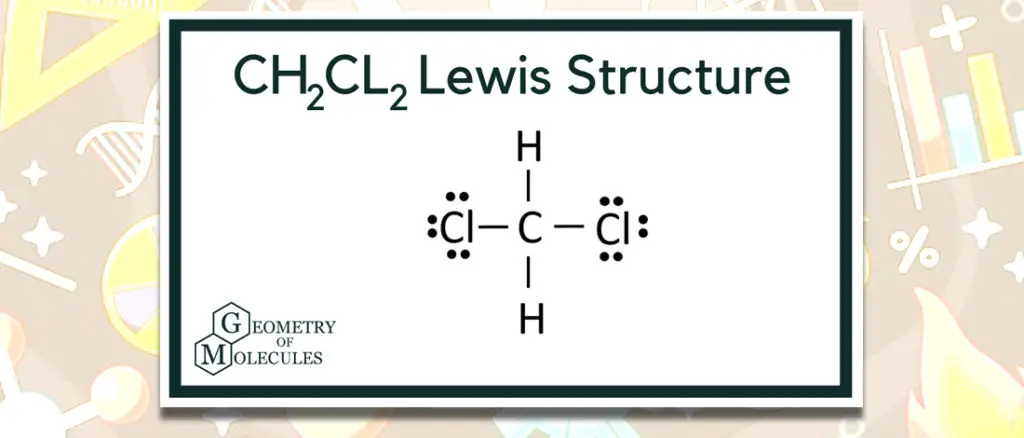
In determining if chemical reactions can affect the structure of a compound, we look at the Lewis Structure [1]. This representation helps us understand the compound’s structure along with eight electrons for it to be stable or inert. In the case of Dichloromethane, Carbon atoms have four valence electrons, Hydrogen atoms have one valence electron, and Chlorine atoms have seven valence electrons. Compared to the electrons of chlorine, such molecules like hydrogen have a lower electronegativity.
The nucleus of a chlorine atom, on the other hand, has 17 electrons around it.
However, Only 7 of the electrons are valence electrons on the outer shell. As a result, there are 20 valence electrons in all. Eight valence electrons established the bond formation out of a total of 20 valence electrons.
How to Determine CH2CL2 Molecular Polarity

CH2CL2 Electronegativity
Polar molecules are molecules where atoms share an unequal proportion of bonded electrons. A higher electronegativity atom pulls the electron pair to its side, gaining a partial negative charge, whereas the other atom acquires a partial positive charge. The electronegativity of an element rises from left to right, according to the periodic table.
Here, you can identify the polarity of Ch2Cl2 by the differences in their electronegativity. In polar molecules, the electronegativity difference ranges from 0.5 to 0.6. the difference in electronegativity between C-H bonds is 0.3, and C-Cl bonds are 0.6. It demonstrates that CH2Cl2 is a moderate polar because the difference in electronegativity between them is quite small.
CH2CL2 Molecular Shape
The shape of a molecule is another aspect that can determine its polarity. The overall center of overlapping positive and negative charges determines whether a complex molecule is a nonpolar compound.
If a molecule forms a symmetric shape, the dipole moments on each molecule cancel out, making the molecule nonpolar. If a molecule’s structure is not symmetrical, it is said to be a polar compound. But no opposite poles are happening in such atoms.
But there’s a possibility that nonpolar molecules have polar bonds, but the dipoles of these polar bonds cancel each other out due to the symmetric form. It simply implies that nonpolar molecules are generated when atoms share a polar bond formation in which their electric charges cancel out. All homonuclear diatomic molecules and all inert gases are considered nonpolar molecules.
Related Posts:
CH2CL2 Dipole Moment
A dipole moment is a significant factor in determining the polarity of a chemical bond between two atoms. In the case of Dichloromethane, the positive and negative charges of all the bonding atoms are distributed as follows: hydrogen = 2.2, carbon = 2.5, and chlorine = 3.1.
Hydrogen molecules, which are way lower than the two bonds, contribute to the unequal distribution of electronegativity. Although hydrogen is less electronegative than a carbon atom, it does not cancel out, resulting in dipole moments in the compound. The more electronegative atom is the one having a partial negative charge in a polar connection. The electron distribution becomes increasingly polarized.
FAQs
CH2Cl2 is a covalent bond. As the molecule creates all the four bonds in the complex, the central carbon atom is hybridized. Chemical reactions happen during the heating of methane and chlorine at high temperatures.
Though Dichloromethane contains hydrogen, Dichloromethane does not contain nitrogen, oxygen, or fluorine. That’s why they are unable to form hydrogen bonds. [2]
So, Is CH2CL2 Polar or Nonpolar?
The intensity of polarity varies differently in other atoms. In CH2Cl2, it is considered a polar molecule because of its tetrahedral structure and the difference in their electronegativity. Its physical properties are as follows: it has a molecular weight of 84.93 g/mol. It has a density of 1.3226g/cm3 and a boiling point of 39.60° Celsius. Also, its melting point is -97.60° Celsius.
Methyl chloride is commonly used to degrease metal surfaces such as railway tracks and equipment and even in the food industry. Even though it is not mixable with water, it is utilized as a solvent in many organic reactions.
Thank you for reading this far! I hope that the information provided in this article will be helpful to you.
References:
- https://courses.lumenlearning.com
- https://www.varsitytutors.com/mcat_physical-help/intermolecular-forces
- 8 Best Books On Evolution (2023 Updated) - May 16, 2022
- How Could Natural Selection Lead To Evolution? (2023) - May 16, 2022
- How Do Fossils Provide Evidence For Evolution? (2023) - May 16, 2022